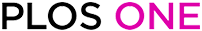Native cysteines and cysteine substitutions in the transmembrane domain of ASIC1a.
A: Model structure of a human ASIC1a subunit based on the chicken ASIC1 crystal structure as published elsewhere [4]: the subunit is made of 2 transmembrane α helices (TM1 and TM2); the cysteines C49, C59 and C61 in the transmembrane α helix 1 (TM1) are shown in green. The substituted cysteines G430C and G433C are in the TM2, and V74C and Y426C are located at the entrance of the channel pore in the extracellular vestibule (ECV). B: Top view of the transmembrane α helices TM1 and TM2 of a single ASIC1a subunit, with the pore lining residues G433C and G430C according to Li et al. 2011. C: Representative recordings of ASIC1a currents elicited at pH 5.5 in the absence (black) or in the presence (red) of 100 μM Cd2+ in xenopus oocytes expressing either ASIC1a wt or the V74C, Y426C, G430C, G433C mutants.